The Science Behind Extreme Water Absorption
Superabsorbent polymers (SAPs) are extraordinary materials capable of absorbing and retaining vast amounts of liquid—often hundreds or even thousands of times their own weight. They are widely used in products such as baby diapers, sanitary napkins, agricultural soil conditioners, medical dressings, and construction materials. Although SAPs are part of everyday life, their working mechanism is not widely understood.
This article explains how superabsorbent polymers work, from their molecular structure to the physical and chemical principles that enable their exceptional absorbency.
Apa Itu Polimer Superabsorben?
Superabsorbent polymers are a class of functional polymers designed to absorb water and aqueous solutions rapidly and retain them under pressure. When exposed to liquid, SAP particles swell dramatically and transform into a soft, gel-like material known as a hydrogel.
Most commercial SAPs are based on cross-linked polyacrylic acid or its sodium or potassium salts. The combination of hydrophilic chemical groups and a three-dimensional polymer network gives SAPs their unique performance.
The Molecular Structure of SAPs
To understand how SAPs work, it is essential to look at their internal structure.
1. Long Polymer Chains
SAPs consist of long-chain molecules formed through polymerization. These chains provide flexibility and space for water molecules to enter.
2. Hydrophilic Functional Groups
Along the polymer chains are hydrophilic (water-loving) groups, such as carboxyl (–COOH or –COO⁻) groups. These groups strongly attract water molecules through hydrogen bonding and electrostatic interactions.
3. Cross-Linking
The polymer chains are lightly cross-linked, meaning they are connected at certain points. Cross-linking prevents the polymer from dissolving in water while still allowing it to swell. The degree of cross-linking directly affects absorption capacity and gel strength.
This unique balance between chain mobility and structural stability is the foundation of SAP performance.
Step-by-Step: How Superabsorbent Polymers Absorb Water
Step 1: Initial Contact with Water
When SAP particles come into contact with water, the hydrophilic groups on the polymer surface immediately begin attracting water molecules.
Step 2: Ionization and Osmotic Pressure
In many SAPs, especially sodium polyacrylate, the carboxyl groups ionize in water, forming negatively charged ions. These charges repel each other, causing the polymer network to expand.
At the same time, osmotic pressure draws water into the polymer network to balance the concentration difference between the inside of the gel and the surrounding solution.
Step 3: Swelling of the Polymer Network
As water enters, the polymer chains stretch, and the SAP particle swells dramatically—sometimes increasing in volume by several hundred times.
Step 4: Formation of a Hydrogel
The absorbed water becomes physically trapped inside the cross-linked network, forming a stable hydrogel. The polymer does not dissolve because the cross-links hold the structure together.
Why SAPs Do Not Leak Under Pressure
One of the most remarkable properties of superabsorbent polymers is their ability to retain water even when pressure is applied.
This happens because:
-
Water is held within the three-dimensional polymer network
-
Hydrogen bonding and ionic interactions keep water molecules associated with the polymer chains
-
Cross-linking prevents the polymer from flowing or dissolving
As a result, SAPs can maintain dryness in applications like diapers and medical pads, even under body weight or movement.
Factors That Affect SAP Performance
Although SAPs are highly effective, their absorption behavior depends on several key factors.
1. Type of Liquid
SAPs absorb pure water most efficiently. Saline solutions, urine, or other electrolyte-containing liquids reduce absorption capacity because dissolved ions weaken osmotic pressure.
2. Degree of Cross-Linking
-
Low cross-linking: Higher absorption, weaker gel
-
High cross-linking: Lower absorption, stronger gel
Manufacturers adjust cross-linking levels depending on the intended application.
3. Particle Size and Shape
Smaller particles absorb liquid faster due to a larger surface area, while larger particles may offer better retention under pressure.
4. Temperature and pH
Extreme temperatures or pH levels can affect polymer structure and absorption behavior, especially in specialized applications.
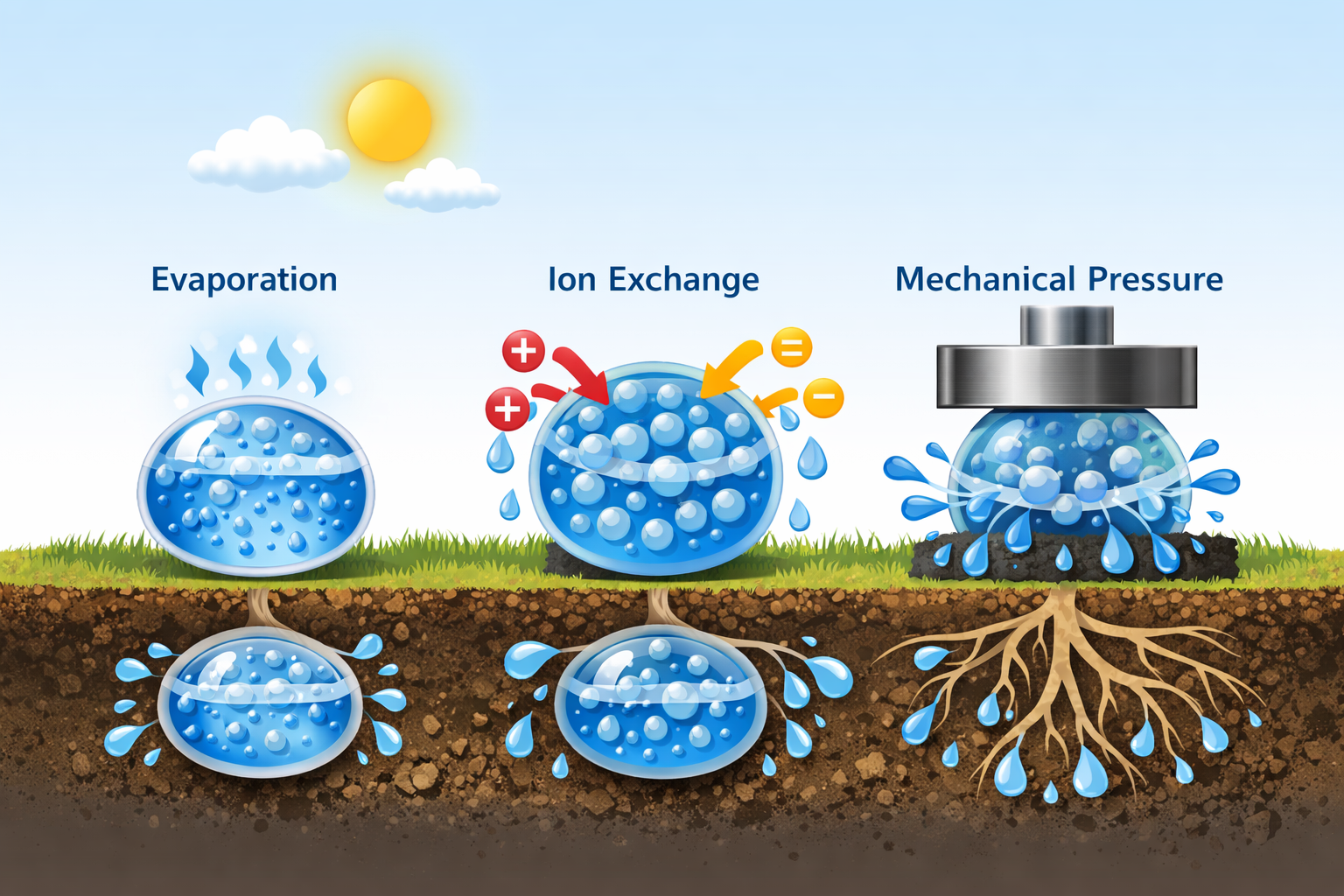
How SAPs Release Water
While SAPs are known for water retention, they can also release water under certain conditions.
-
Evaporation: In open environments, water slowly evaporates from the gel
-
Ion exchange: Changes in surrounding ion concentration can cause water release
-
Mechanical pressure: Extreme pressure can force some water out
This controlled release capability is especially valuable in agriculture, where SAPs act as water reservoirs for plant roots.
How Different SAP Formulations Work
Sodium-Based SAPs
Sodium polyacrylate is the most common SAP used in hygiene products. It offers high absorption capacity and fast swelling.
SAP Berbasis Kalium
Potassium-neutralized SAPs are often used in agriculture because potassium is beneficial to plant growth.
Copolymer SAPs
SAPs made from acrylic acid and acrylamide provide improved strength and performance in soil and construction applications.
Comparison with Traditional Absorbent Materials
Traditional absorbents like cotton, paper, and sponges work through capillary action, where liquid fills empty spaces between fibers. Once these spaces are full, absorption stops, and liquid can easily be released under pressure.
In contrast, SAPs chemically bind water at the molecular level, enabling far greater absorption and retention.
Safety and Environmental Considerations
Commercial SAPs are generally non-toxic and safe for consumer use. They are widely regulated and tested, especially in hygiene and medical products.
Environmental research is ongoing to develop:
-
Bio-based SAPs
-
Biodegradable alternatives
-
More sustainable manufacturing processes
These innovations aim to reduce environmental impact while maintaining performance.
Real-World Examples of SAP in Action
-
Diapers: SAPs absorb urine and lock it away, keeping skin dry
-
Pertanian: SAPs store water in soil and release it to plants during dry periods
-
Medical dressings: SAPs absorb wound exudate while maintaining a moist healing environment
-
Concrete curing: SAPs release water gradually to prevent cracking
The Future of Superabsorbent Polymer Technology
As material science advances, SAP technology continues to evolve. Researchers are developing SAPs with smarter responsiveness, such as materials that react to temperature, pH, or pressure. These next-generation hydrogels could revolutionize medicine, agriculture, and environmental protection.
Kesimpulan
Superabsorbent polymers work through a carefully engineered combination of chemistry and physics. Their hydrophilic functional groups attract water, osmotic pressure drives absorption, and cross-linked networks trap liquid in a stable hydrogel. This unique mechanism allows SAPs to outperform traditional absorbent materials by a wide margin.
From everyday hygiene products to advanced industrial and medical applications, SAPs demonstrate how polymer science can solve real-world problems efficiently and sustainably.

