Superabsorbent polymers (SAPs) are materials that have the remarkable ability to absorb and retain large amounts of liquid relative to their own mass. They are commonly used in products such as diapers, sanitary pads, and wound dressings due to their excellent absorbing properties.
The mechanism behind how superabsorbent polymers work involves several key factors:
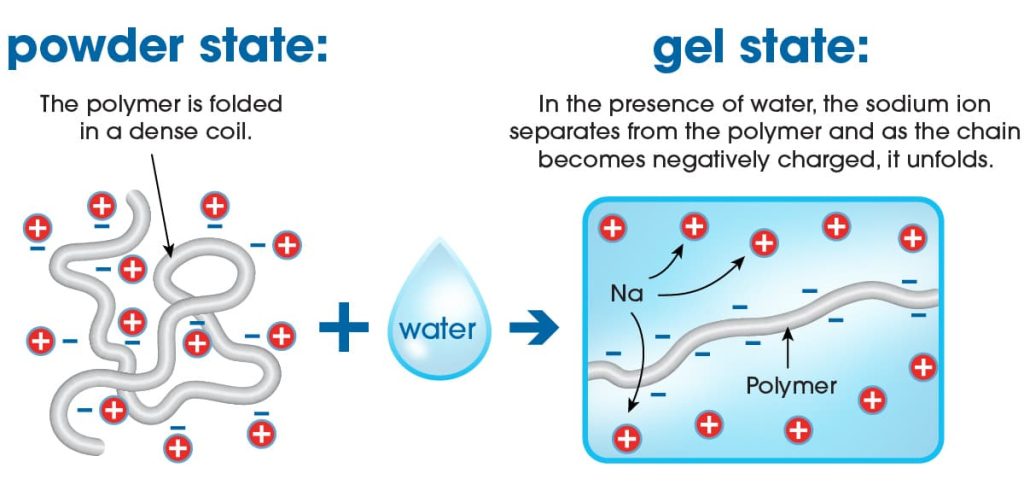
- Chemical Structure: Superabsorbent polymers are typically cross-linked hydrophilic polymers, meaning they have a network of interconnected molecules that are attracted to water (hydrophilic).
- Ionic Groups: These polymers contain ionic groups, such as carboxylate (-COO-) or sulfonate (-SO3-) groups, that can attract and bind water molecules through hydrogen bonding and electrostatic interactions.
- Swelling Capacity: When exposed to water or other aqueous solutions, superabsorbent polymers undergo rapid swelling due to the influx of water molecules into the polymer network. This swelling process is driven by osmotic pressure and capillary forces within the polymer structure.
- Absorption Mechanisms: There are primarily two absorption mechanisms involved:
- Swelling: Water penetrates into the polymer network, causing it to swell and expand. This swelling can occur many times the original dry volume of the polymer.
- Gel Formation: As water enters the polymer, it forms a gel-like substance within the polymer matrix, trapping the water molecules and preventing them from easily escaping.
- Retention: Once absorbed, the water is retained within the polymer structure even under pressure. This retention ability is crucial for applications like diapers, where the absorbed liquid should not leak even when the diaper is compressed.
- Absorption Rate and Capacity: Superabsorbent polymers can absorb water rapidly, often within seconds to minutes, depending on the specific formulation. They can typically absorb many times their weight in water, making them highly effective for moisture management applications.
Overall, superabsorbent polymers work by utilizing their unique chemical structure and swelling properties to absorb and retain significant amounts of liquid, making them invaluable in various industries where moisture control is essential.

