The Inventor of Potassium Polyacrylate
Potassium polyacrylate, a superabsorbent polymer, was developed in the early 1970s as part of ongoing research into materials that could improve water retention in soils and other applications. The invention of potassium polyacrylate is attributed to advancements in polymer chemistry by multiple researchers and organizations. It built on the foundational work done with sodium polyacrylate, incorporating potassium ions to create a variant with distinct properties.
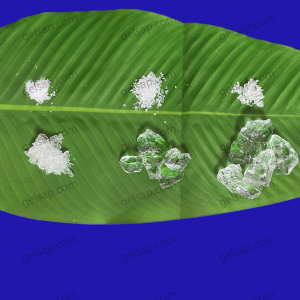
Why Does Potassium Polyacrylate Absorb Water?
Potassium polyacrylate absorbs water due to its unique chemical structure. It consists of long chains of acrylate monomers, each containing a carboxyl group that is ionized in the presence of potassium ions. These carboxyl groups attract water molecules through hydrogen bonding and electrostatic forces. When exposed to water, the polymer chains expand and create a network that traps water molecules, leading to significant swelling and water retention. This is driven by the polymer’s hydrophilic nature and the osmotic pressure created by the dissociated potassium ions.
Why Is Potassium Polyacrylate Absorbent?
The absorbency of potassium polyacrylate is a result of its cross-linked polymer structure, which forms a network of spaces that can hold water. The potassium ions (K+) within the polymer enhance its water absorption capacity by increasing the osmotic pressure when they dissociate in water. This causes the polymer to swell and retain large volumes of water, much more than its own weight. The cross-linking also ensures that the polymer maintains its structural integrity while absorbing water, preventing it from dissolving and allowing it to form a gel-like substance.
Why Is Potassium Polyacrylate Used in Diapers?
While potassium polyacrylate is not as commonly used in diapers as sodium polyacrylate, it shares similar properties that make it a suitable absorbent material. Its ability to absorb and retain large amounts of water helps keep the skin dry and prevent leaks, which is crucial in diaper applications. When incorporated into the core of a diaper, potassium polyacrylate quickly absorbs urine and transforms it into a gel, maintaining comfort and dryness for the wearer. This reduces the risk of diaper rash and other skin irritations.
Can Potassium Polyacrylate Be Recycled?
Recycling potassium polyacrylate presents challenges similar to those faced with other superabsorbent polymers. Its complex chemical structure and the contamination it often encounters after use make recycling difficult. However, there are several potential methods being explored:
- Mechanical Recycling: This involves physically separating and processing used materials to recover potassium polyacrylate for reuse. This method can be labor-intensive and costly, but it holds promise for reducing waste.
- Chemical Recycling: Chemical processes can break down potassium polyacrylate into its monomers or other reusable components. While still under development, this approach could provide a sustainable way to recycle the polymer.
- Biodegradation: Research is ongoing into the use of specific microorganisms or enzymes that can degrade potassium polyacrylate. Although in experimental stages, this method could offer a future solution for environmentally friendly disposal.
In conclusion, potassium polyacrylate is a versatile material with significant applications in water absorption and retention, including its use in hygiene products like diapers. Its invention and development have provided effective solutions for moisture management. Although recycling remains a challenge, ongoing research aims to find sustainable ways to manage and reuse this superabsorbent polymer, contributing to environmental conservation efforts.

